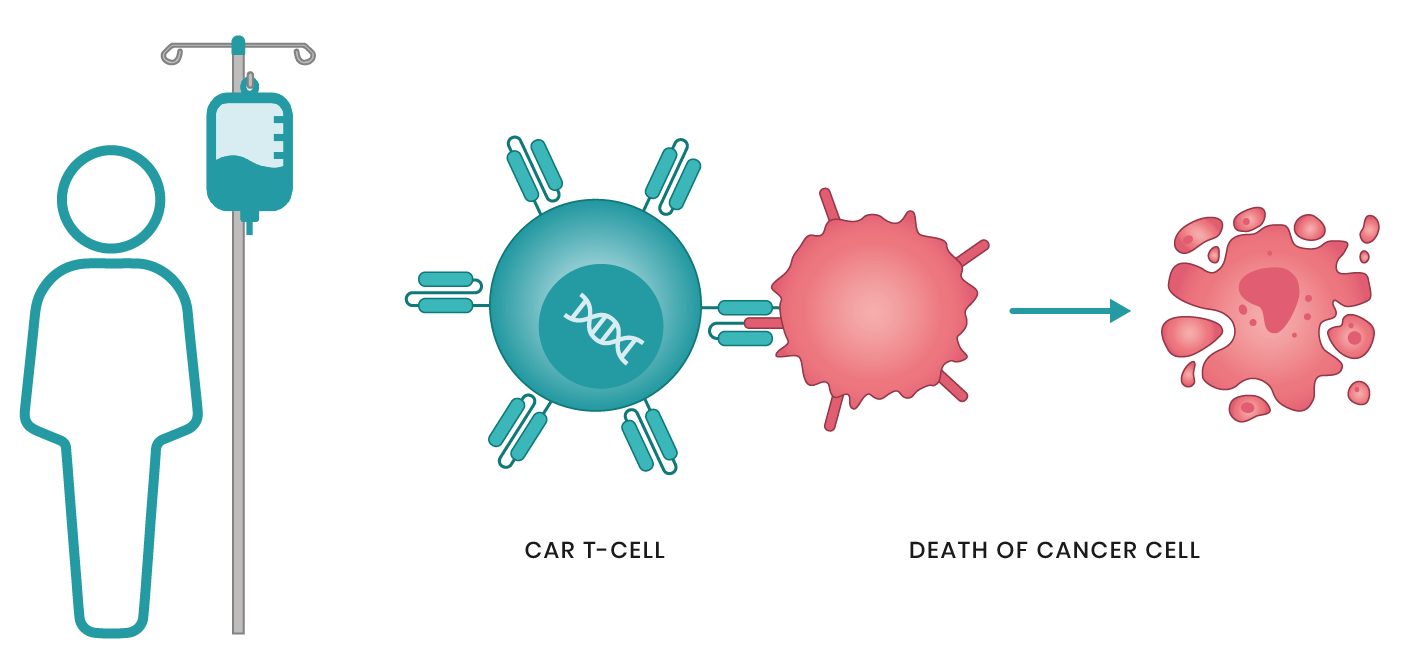
In the battle against cancer, the immune system is often our most powerful ally. But for decades, scientists struggled to harness its full potential. Enter CAR T cell therapy—a groundbreaking form of immunotherapy that has not only defied expectations but also rewritten the future of cancer treatment. Imagine a world where your body’s own immune cells are engineered to hunt down and destroy cancer, offering a potential cure for diseases once considered untreatable. This is no longer science fiction. CAR T cell therapy is making that vision a reality, with dramatic successes in blood cancers and an expanding arsenal aimed at solid tumors. But with great potential comes new challenges. What does the future hold for this cutting-edge treatment? Let’s dive into the history, innovations, and the promise of next-generation CAR T cell therapies.
CAR stands for Chimeric Antigen Receptor, a synthetic receptor that is engineered to be present on the surface of T cells (or other immune cells) in CAR T cell therapy. The purpose of the CAR is to recognize and bind to specific antigens present on the surface of cancer cells, thereby activating the T cell to attack and destroy the tumor.
A chimeric receptor means that the CAR is a hybrid (or fusion) of different parts that don’t naturally occur together. Here’s a breakdown of the key components of a CAR:
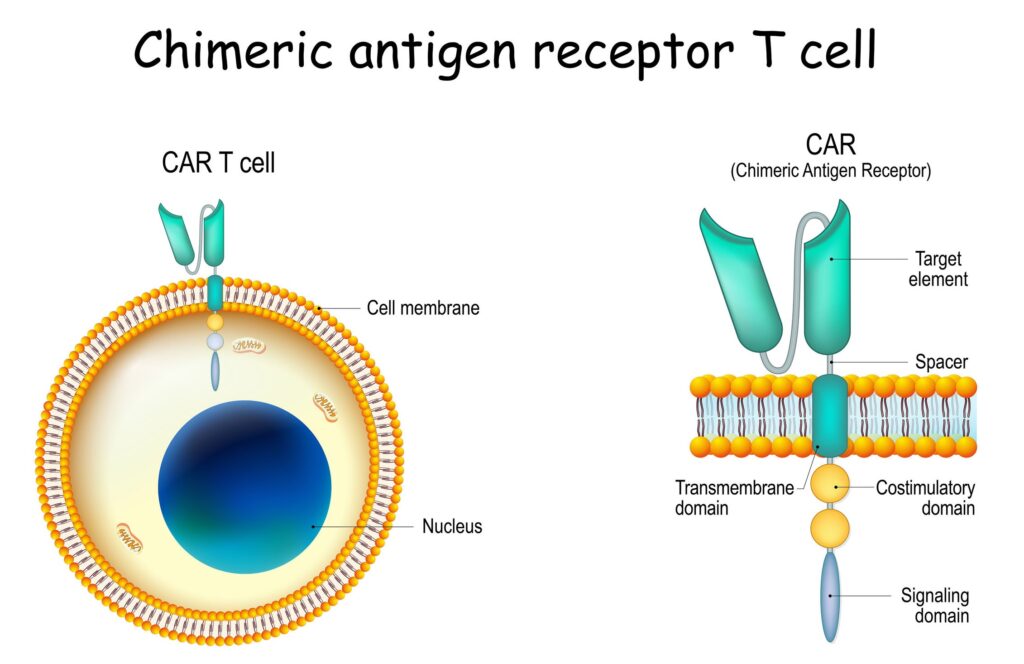
- Extracellular antigen-binding domain: This part of the CAR is designed to recognize and bind to a specific antigen on the surface of cancer cells. This domain is often derived from the single-chain variable fragment (scFv) of an antibody, which is the part of an antibody that binds to its target.
- Transmembrane domain: This anchors the CAR to the T cell’s surface, allowing the receptor to integrate into the cell membrane.
- Intracellular signaling domain: Once the CAR binds to the target antigen on a cancer cell, this domain transmits a signal inside the T cell to activate it, enabling the T cell to attack the cancer cell. The signaling domain typically includes molecules like CD3ζ and costimulatory molecules like CD28 or 4-1BB, which enhance the T cell’s activation and persistence.
History of CAR T-cell Therapy
CAR T cell therapy has a rich history that spans several decades:
- 1980s – Initial Concepts: The first ideas for engineering T cells to fight cancer began in the 1980s. Early research focused on the idea of genetically modifying immune cells to express antibodies that could specifically target cancer cells.
- 1990s – Development of the First CARs: In the early 1990s, scientists developed the first chimeric antigen receptors. These early CARs were simple and based on antibody fragments fused to signaling domains. While these early CARs showed promise in preclinical models, their effectiveness was limited.
- 2000s – Optimizing CARs: Over the next decade, researchers worked to improve the design of CARs by adding costimulatory domains (such as CD28or4-1BB) to increase T cell activation and persistence. This period also saw improvements in T cell engineering techniques, including viral vectors to deliver the CAR genes more efficiently.
- 2010s – Clinical Success and Approval: CAR T-cell therapy began to show substantial clinical success, particularly in treating hematologic malignancies like leukemia and lymphoma. In 2017, the FDA approved the first CAR T-cell therapy, Kymriah (tisagenlecleucel), for acute lymphoblastic leukemia (ALL) in pediatric and young adult patients. This was followed by approvals for Yescarta (axicabtagene ciloleucel) for large B-cell lymphoma and other CAR T therapies.
- Present and Future: Today, CAR T-cell therapy is used in clinical practice for certain blood cancers, and there is active research to improve the therapy and expand its use to solid tumors, as well as to enhance its safety profile, persistence, and efficacy.
Next-Generation CAR T-cell Therapies
As researchers continue to improve CAR T-cell therapy, several next-generation CARs are being developed with the aim of overcoming the limitations of earlier versions. Here’s a look at some of the key innovations and their associated pros and cons:
1. Dual-target CARs (or Bispecific CARs)
These CARs are designed to recognize two different antigens on cancer cells, which can help reduce the risk of antigen escape (when cancer cells lose the antigen targeted by the CAR).
2. Armored CARs
Armored CAR T cells are engineered to secrete additional factors that enhance their activity or counteract the tumor’s immune-suppressive environment. For example, CAR T cells can be engineered to produce cytokines (like IL-12) or checkpoint inhibitors (like anti-PD-1) to boost immune responses.
3. Memory CAR T-cells (or Stem-like CAR T-cells)
These CAR T cells are engineered to have a memory phenotype, meaning they have a long-lived, stem-cell-like characteristic that enables them to persist in the body for longer periods and respond to cancer recurrence.
4. Off-the-Shelf CAR T-cells (Allogeneic CAR T-cells)
These CAR T cells are derived from healthy donors rather than from the patient’s own cells. The goal is to make CAR T therapy more widely available and reduce the need for personalized treatment.
5. Solid Tumor-Specific CARs
Many next-generation CAR T therapies are being designed to target antigens on solid tumors, which are more difficult to treat than hematologic cancers due to issues like the tumor microenvironmentand immune suppression.
Summary: Pros and Cons of Next-Generation CAR T-cells
| Pros | Cons |
| Dual-target CARs: Reduced risk of antigen escape. | Increased complexity and technical difficulty. |
| Armored CARs: Enhanced immune activation. | Risk of uncontrolled cytokine release and toxicity. |
| Memory CARs: Longer persistence and durability. | Technical challenges in manufacturing and expanding. |
| Off-the-shelf CARs: Faster availability and lower cost. | Risk of graft-versus-host disease and immune rejection. |
| Solid Tumor CARs: Potential for treating solid tumors. | Tumor microenvironment and antigen heterogeneity challenges. |
The future of CAR T-cell therapy is promising, with these next-generation improvements aiming to overcome current limitations and broaden its applications. However, many of these therapies are still in the experimental or clinical trial stages, and ongoing research is necessary to address safety, efficacy, and manufacturing challenges.